Forced Exercise and Stress Reduction Reverse Parkinson’s Symptoms
If you read last week’s post Doctors Call for an End to the Parkinson’s Pandemic, you learned that Parkinson’s disease is most often the result of a complex interplay between genetic and environmental factors, which include exposure to neurotoxic chemicals, head trauma, lack of exercise, diet, gut dysbiosis, and chronic stress.
While there is no cure for Parkinson’s disease, there are documented cases of improvement and recovery. Recovery in these cases is defined mainly by an elimination of motor symptoms. As I mentioned in last week’s post, motor symptoms begin when dopamine levels and neuron loss reach a critically low threshold—I’ll explain how that works in this post. When people are able to boost their dopamine levels and/or restore enough dopaminergic neurons that they can get past this threshold, their motor symptoms can go away.
In this post I’ll discuss why motor symptoms occur, research on neurogenesis in the substantia nigra of Parkinson’s patients, how exercise (especially forced exercise) and stress reduction reduce motor symptoms, and stories of recovery from Parkinson’s disease. As you will learn, reducing and potentially eliminating motor symptoms—and even restoring sense of smell—is a realistic goal that Parkinson’s patients are already pursuing.
Motor symptoms occur when the “NoGo” pathway is activated
The motor symptoms of Parkinson’s disease typically appear years after the disease process has begun—often 20 or more years after. It’s estimated that motor symptoms appear when approximately 30% to 60% of dopaminergic (dopamine-producing) neurons in the substantia nigra are lost. Studies show varied results when it comes to the actual percentage of substantia nigra neuron loss necessary to produce motor symptoms, and it’s safe to say that the exact percentage is different from person to person. Regardless of the percentage, it is understood that when dopamine levels decrease to a critical threshold, tremor or other motor symptoms may be felt—sometimes suddenly. For many people, this is the first noticeable sign of the disease.
Dopaminergic neurons in the substantia nigra send dopamine into two basal ganglia motor loops, referred to as the direct pathway of movement and the indirect pathway of movement. Dopamine regulates motor activity by acting on dopamine receptors, of which there are two types: D1-like receptors are present in the direct pathway, and D2-like receptors are present in the indirect pathway (if you read more about this topic, you’ll hear about these receptors).
The direct pathway allows us to move in ways that we want to; activation of the direct pathway increases ease of movement and of initiating movement. In contrast, the indirect pathway allows us to suppress unwanted movement.
The direct pathway is referred to as the “Go” pathway, while the indirect pathway is referred to as the “NoGo” pathway. When the Go pathway is activated, we move easily; when the NoGo pathway is activated, movement is suppressed.
Low or fluctuating levels of dopamine, which occur in Parkinson’s disease as dopaminergic neurons die off, weaken the direct pathway and strengthen the indirect pathway. When dopamine levels fall to a critical threshold, tremor or other motor symptoms may occur, sometimes suddenly, as the indirect pathway is activated.
Neurogenesis in the substantia nigra, and promising stem cell treatments
In last week’s post I explained that Parkinson’s disease is a degenerative neurological condition in which dopaminergic (dopamine-producing) neurons in a part of the brain called the substantia nigra die off. Other parts of the brain suffer neurodegeneration as well, causing some of the non-motor symptoms of Parkinson’s. I’ll now discuss promising research about neurogenesis (production of new neurons) as it applies to Parkinson’s disease; if you’re not familiar with neurogenesis, I encourage you to read this post.
In 2003, scientists in Sweden demonstrated that neurogenesis occurs in the substantia nigra of adult mice. Their research showed that the type of dopaminergic neurons lost in Parkinson’s disease are actually regenerated throughout life. While the rate of neurogenesis in the substantia nigra is slower than in the hippocampus, if the rate of neural turnover is constant, the entire population of dopaminergic neurons in the substantia nigra could be replaced during the lifespan of a mouse. The study showed that not only does neurogenesis in the substantia nigra occur, but the newborn neurons are then integrated into neural circuits.
The discoveries of this study imply that “disturbances in the finely tuned equilibrium of cell genesis and cell death could result in neurodegenerative disorders.” The researchers suggest that Parkinson’s disease could in some cases be caused by decreased neurogenesis rather than increased cell death. Another explanation they suggest is that neurogenesis in Parkinson’s patients can’t keep up with the increased rate of cell death caused by Lewy bodies.
In 2016, researchers at Boise State University in Idaho discovered that dopaminergic neurons are replenished in adult mouse models of Parkinson’s disease. The researchers created a chronic, systemic inflammatory state in mice’s brains to simulate that which occurs in Parkinson’s disease. Their results indicate that inflammation may inhibit neurogenesis in the substantia nigra, leading or contributing to the net loss of neurons in Parkinson’s disease. The researchers note that neurogenesis has been difficult to prove due to limitations of current cell lineage tracing methods, and they were able to demonstrate neurogenesis of nigral neurons using a new tracing model that they developed.
In 2011, neuroscientists in the Netherlands studied the brains of 25 people: 10 with Parkinson’s disease, 10 healthy controls, and 5 with Lewy body disease (the presence of Lewy bodies, but no clinical symptoms of Parkinson’s disease). The researchers found neural stem cells in the subventricular zone of every donor, with no significant differences in number between the three groups. They cultured neural stem cells from the Parkinson’s patients and confirmed that the cells were viable. While this was a small study, having proof that viable neural stem cells are produced in the brains of Parkinson’s patients is extremely encouraging.
An article in the Journal of Experimental Neuroscience states: “To compensate the degenerative rate of DAergic neurons, we have only 2 choices, either enhance the formation of newborn neurons and endogenous regenerative capacity or reduce the death rate of existing neurons.” Many scientists are pursuing the first route—exploring ways to enhance our natural process of producing neural stem cells to replace the dopaminergic neurons that are lost in Parkinson’s disease.
In 2012, researchers in South Korea injected human stem cells into mice with Parkinson’s disease. The stem cells increased neurogenesis in the subventricular zone and the substantia nigra, which led to an increase in the number of neural precursor cells that turned into dopaminergic neurons in the substantia nigra. The researchers suggest that this approach of enhancing endogenous neurogenesis to repair the damaged Parkinson’s brain could have a significant impact on future strategies.
However, scientists in the Netherlands and Australia favor non-invasive approaches. They caution that transplanting neural stem cells has both ethical and immunological challenges. When doing stem cell transplants, a donor is necessary, and the patient’s immune system must be suppressed in order to prevent rejection. These scientists prefer non-invasive treatment approaches that stimulate neurogenesis and mobilize endogenous neural stem cells—those that are naturally produced in the brain—to survive, migrate, and differentiate into dopaminergic neurons in the substantia nigra.
One such approach is being explored by neuroscientists at the Karolinska Institute in Sweden. By injecting transcription factors (proteins that regulate genes) into mice with Parkinson’s, they can turn astrocytes (an abundant type of brain cell that provide support and maintain homeostasis) into dopaminergic neurons. Five weeks after receiving treatment, the mice with Parkinson’s were walking normally. This “direct reprogramming of brain cells has the potential to become a novel therapeutic approach for Parkinson’s.”
One of the study authors notes that the reprogrammed cells would probably be damaged by whatever caused Parkinson’s in the first place. In cell transplants for other health conditions, disease tends to catch up with transplanted cells in 15-20 years. Treatments to enhance endogenous neurogenesis will likely buy Parkinson’s patients important time, but they may need to be repeated.
Until and even after these treatments become approved for human use, it is extremely important for Parkinson’s patients to understand how to enhance neurogenesis naturally. Exercise and stress reduction are two of the best ways to encourage neural stem cells to survive, migrate, and differentiate into the type of neurons that the brain needs. Parkinson’s patients are already using exercise and stress reduction to repair their damaged brains, and the result is a reduction or elimination of motor symptoms, and even restoration of sense of smell.
For Parkinson’s patients, exercise does what medication can’t
We learned last week that exercising regularly helps to prevent Parkinson’s. In a study of more than 7,300 male veterans, those with the lowest level of physical fitness had a 316% higher risk of developing Parkinson’s than those with the highest level of physical fitness. And a meta-analysis of more than half a million participants found a dose-response relationship: The more moderate to vigorous physical activity you do, the more your risk of getting Parkinson’s is reduced.
Reduced physical activity is likely an early symptom of Parkinson’s disease. But neuroscientists at the University of Texas in Austin did an interesting study to explore whether or not a reduction in physical activity also speeds up the degeneration of dopaminergic neurons. By restricting the movement of rats, they were able to show that the restriction of movement led to severe and chronic loss of dopaminergic neurons and impaired motor function. Neurons that aren’t used tend to wither away and die—this is called neural pruning. When it comes to movement, the principle of “use it or lose it” applies not only to our muscles, but to the health of our brain as well.
So we know that exercise is protective against Parkinson’s—great. If you already exercise, keep doing it, and if you don’t, start. But what about if you already have Parkinson’s?
A 2017 study at the Cleveland Clinic in Ohio had exciting results. Using fMRI scans, researchers found that forced exercise (which I’ll describe in the next section) and Parkinson’s medications had very similar effects on the brain. Forced exercise also improved participants’ scores on the Unified Parkinson’s Disease Rating Motor Scale by 50%. The researchers explain that “Both levodopa therapy and forced exercise are thought to increase the amount of available dopamine within the dorsolateral striatum.” They also note that exercise has little expense and none of the negative side effects of medication that can compromise quality of life for Parkinson’s patients.
Likewise, a team of researchers from the U.S., China, and Spain agree that “exercise is a universally available, side effect-free medicine that should be prescribed to vulnerable populations as a preventive measure and to Parkinson’s disease patients as a component of treatment.” They note that there is currently no pharmacological treatment that can modify or slow the disease or protect dopaminergic neurons—for Parkinson’s patients, exercise does what medication can’t.
The main way that exercise stimulates neurogenesis and keeps the brain healthy is by boosting production of neuroprotective growth factors, one of which is brain-derived neurotrophic factor (BDNF). BDNF is a protein that acts like Miracle-Gro for brain cells: it stimulates the growth of new neurons, helps them survive, and encourages the formation of new synapses. Neurotrophic factors increase neurogenesis in the substantia nigra, and may help to recover the phenotype of dopaminergic neurons or increase sprouting of dopaminergic axons. Studies have also shown that exercise reduces damage to dopaminergic neurons in motor circuits, preserves dopamine levels in Parkinson’s animal models, and reduces cellular inflammation and oxidative stress.
Parkinson’s patients who exercise more than 3 times per week for a total of more than 180 minutes have a more robust dopamine system and more dopamine release in response to exercise when compared to Parkinson’s patients who exercise less often or for shorter durations. They also experience less bradykinesia, better function, and less apathy.
Running can completely protect mice from the neurotoxic effects of MPTP, a chemical that kills dopaminergic neurons in the substantia nigra, causing Parkinson’s disease. Researchers found that one month of voluntary running on a running wheel provided no protection, two months provided partial protection, and three months provided complete protection of loss of dopaminergic neurons.
In addition to protecting against the loss of neurons, running has been shown to increase the number of dopaminergic neurons in animal models of Parkinson’s. Researchers observed restoration of lost neurons after 18 weeks of treadmill running in mice treated with MPTP. And researchers in Korea found that running for 30 minutes twice a day for just 16 days was enough to recover some of the dopaminergic neurons killed by the neurotoxin 6-OHDA.
Dr. Jay Alberts discovers the magic of forced exercise for Parkinson’s patients
In 2003 Dr. Jay Alberts rode the Des Moines Register’s Annual Great Bike Race Across Iowa with his friend Cathy, who has Parkinson’s disease. Dr. Alberts rode the front of a tandem bicycle with Cathy pedaling behind him. Cathy remarked, “for this week it did not feel like I had Parkinson’s.” After several days of riding, Dr. Alberts noticed that Cathy’s motor control, most notably her handwriting, had improved. This set him on a path toward groundbreaking research on the benefits of forced exercise for Parkinson’s patients.
When the effects of exercise are tested in animal models of Parkinson’s, the exercise is often “forced,” meaning that the animal is forced to exercise at a more intense level than they would choose to on their own. Researchers have found that this forced exercise (FE) is far more effective than voluntary exercise in stimulating the release of neurotrophic factors and enhancing neurogenesis. Researchers suggest that “contradictory results in human and animal studies are caused by differences between voluntary (human) versus forced exercise (animal)…patients with PD may not be able to exercise (voluntarily) at sufficiently high rates to trigger the endogenous release of the neurotrophic factors thought to underlie global improvements in motor function.”
Dr. Alberts realized that in riding on the back of a tandem bike with him, Cathy was doing forced exercise—pedaling faster than she would have on her own. So in 2009, he and other researchers at the Cleveland Clinic tested the effects of forced exercise versus voluntary exercise for Parkinson’s patients. Patients completed eight weeks of either forced exercise or voluntary exercise on stationary bicycles. Patients in the forced exercise group were forced to pedal at a speed 30% faster than their preferred voluntary speed. While cardiovascular fitness improved for both groups, the forced exercise group showed a 35% improvement in motor function, while the voluntary exercise group had no improvement.
An exciting aspect of the study’s findings was the fact that while the forced exercise used only lower-body muscles, motor control improved in manual dexterity tasks using the upper body. This meant that the forced exercise was not just improving muscular control or strength locally in the muscles that were exercised; it was improving motor function by affecting the brain in some way.
And remarkably, three participants in the study noticed that their sense of smell had returned. One noted that he could smell diesel fuel for the first time in 10 years, another walked into his house and smelled onions, and the third suddenly realized that he had body odor. These observations led the researchers to start tracking improvements in olfaction (sense of smell), the loss of which is often a very early symptom of Parkinson’s. They found that improvements in sense of smell correlated with pedaling speed; participants who pedaled faster than 83 RPM tended to have improvements in olfaction.
Dr. Alberts and his team then carried out a study which I mentioned earlier. In this study, forced exercise improved motor function by 50%. The researchers used fMRI scans to see how exercise affected the participants’ brains, and as you can see below, they found that forced exercise and medication activate the same brain areas. The researchers explain: “Both levodopa therapy and forced exercise are thought to increase the amount of available dopamine within the dorsolateral striatum.”
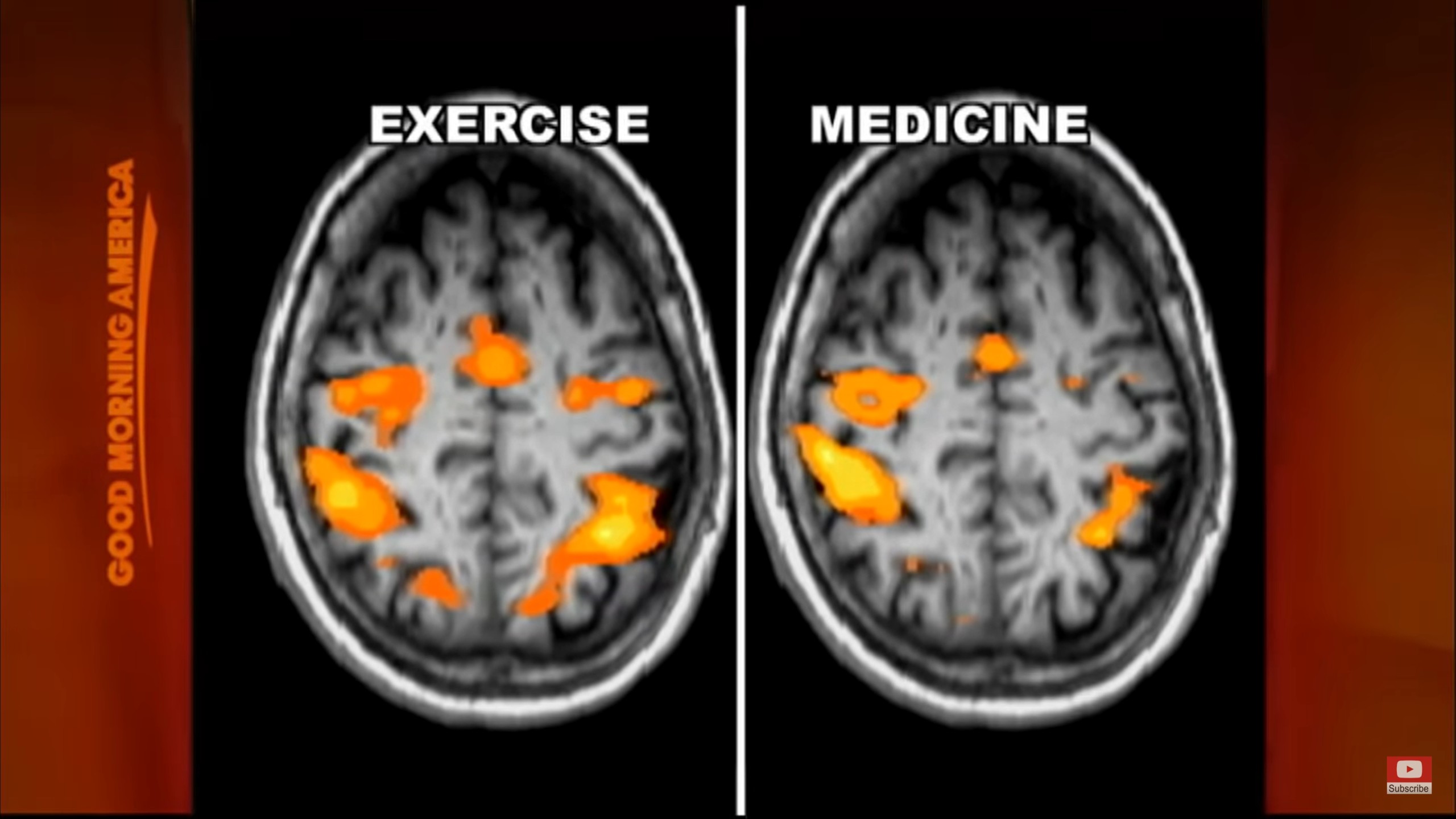
From Good Morning America.
Dr. Alberts and his team then carried out a trial with 100 Parkinson’s patients. They found that “an 8-week high intensity aerobic exercise program significantly improved motor function scores, turning and gait speed, and information processing.” And somewhat surprisingly, the positive results continued for up to eight weeks after the participants stopped exercising, showing that the exercise had led to lasting changes in brain function.
In May 2019, Dr. Alberts was awarded a five-year, $3 million grant to conduct a clinical trial studying the long-term effects of high-intensity aerobic exercise on Parkinson’s disease. The trial will track the progress of 250 Parkinson’s patients as they participate in the exercise program for a full year. This is very exciting because previous trials, as well as other studies of interventions for Parkinson’s disease, have typically been carried out over very short time frames. When dealing with a disease that has been in progress for decades, testing an intervention for just eight weeks is limiting.
Participants in this latest study use Peloton indoor bikes at home, are instructed to exercise three times per week for 12 months, and have their motor function evaluated every three months throughout the year. The study is in its first year right now, recruitment of participants is ongoing, and results may be published within the next few years. The researchers hope this will lead to patient-specific exercise prescriptions that take into account individual disease variables and can estimate potential outcomes. Since forced exercise has comparable effects as medications and deep brain stimulation on improving motor function, Dr. Alberts says “exercise is medicine” for Parkinson’s patients.
If you’re interested in this topic, you may enjoy watching Dr. Alberts describe his research in a talk he gave for the Davis Phinney Foundation for Parkinson’s. Dr. Alberts makes a number of important points, one of which is that when doing forced exercise on a tandem bicycle, the Parkinson’s patient must be actively involved and contributing to the movement. If the patient is passive, they do not experience improvement in motor function.
In addition, the exercise does not have to be cycling. Dr. Alberts suggests that a treadmill or stationary rowing machine could be used as well—the most important aspect of the exercise is that the person must be exercising at a faster rate than they would choose to do on their own. He recommends reducing resistance so that speed can be increased.
Dr. Alberts and Cathy Frazier, his friend whose experience first inspired his research, created Pedaling for Parkinson’s, a group indoor cycling class that is now held all over the country. If you’re interested in trying this approach, check to see if there’s a Pedaling for Parkinson’s class near you!
Dr. Alberts appears in the documentary Ride with Larry, an inspiring film that follows Parkinson’s patient Larry Smith as he does a 300-mile bike ride across South Dakota. The film also offers a helpful introduction to the use of medical marijuana, which Larry finds very helpful in relieving his Parkinson’s symptoms.
A final note on exercise: Vigorous, forced exercise is not the only type of exercise that Parkinson’s patients should do; they can also benefit greatly from types of movement that involve building strength, coordination, and balance. For example, yoga improves motor function, postural stability, functional gait and freezing gait, and reduces the risk of falling in Parkinson’s patients. Similarly, Tai Chi reduces risk of falling and improves motor function in Parkinson’s patients.
Practicing forced aerobic exercise in addition to other types of movement that you enjoy is an ideal combination. Dr. John Ratey, author of Spark: The Revolutionary New Science of Exercise and the Brain, recommends doing both aerobic exercise and activities that demand focus and coordination, like martial arts, dance, rock climbing, and yoga, in order to fully stimulate your brain.
Mindfulness, meditation, and stress reduction reduce Parkinson’s symptoms and boost dopamine
If you read last week’s post, you learned why chronic stress is a risk factor for Parkinson’s disease. Chronic stress causes neuroinflammation, oxidative stress, and elevated cortisol levels, which lead to a loss of dopaminergic neurons in the substantia nigra. Chronic stress also decreases neurogenesis. Stressful life events, job-related stress, and stress-related personality traits all increase the risk of developing Parkinson’s. Stress level also worsens motor symptoms and predicts worse disease progression.
The idea that chronic stress is a factor in developing Parkinson’s has become mainstream fairly recently. Studies have shown how stress leads to the death of dopaminergic neurons, but not much research has been done yet to test how methods of stress reduction may improve symptoms, and those that have been done have been short-term. However, in personal reports of improvement of motor symptoms and recovery, nearly everyone cites mindfulness, mindful movement, meditation, and/or stress reduction as a critical part of their recovery—you’ll read some of these stories in the next section.
In 2013, scientists in Belgium carried out the first study to test the neurobiological effects of mindfulness in Parkinson’s patients. After eight weeks of a mindfulness practice, the patients had increased gray matter density (number of neurons) in neural networks that play a role in Parkinson’s disease. In a separate study, the same researchers found that an eight-week mindfulness program led to a significant improvement in motor symptoms.
Neurologists in Israel tested the effects of relaxation guided imagery on tremor in 20 Parkinson’s patients. Relaxation guided imagery dramatically decreased tremor in all patients, and in 15 of the 20 patients, tremor was completely eliminated for 1-13 minutes. Patients reported improvements lasting 2-14 hours afterward. In comparison, listening to relaxing music also reduced tremor but to a lesser degree, and self-relaxation had no significant effect on tremor.
In 2019, researchers in Hong Kong explored how mindfulness yoga compares with stretching and exercise training for people with Parkinson’s. Mindfulness yoga combines movements that improve balance and coordination with methods of stress release, relaxation, awareness, and focus. The study found that yoga had comparable benefits to stretching and resistance training in terms of motor symptoms and mobility, and that yoga had the added benefits of reducing psychological distress and improving spiritual well-being and health-related quality of life. Similarly, the Feldenkrais Method of somatic education improves quality of life scores and reduces depression in Parkinson’s patients.
In 2001, researchers demonstrated for the first time how a conscious experience is associated with the release of a neurotransmitter. During Yoga Nidra meditation, which reduces stress and is known as “yogic sleep,” participants experienced a 65% increase in endogenous dopamine release.
And in 2013, researchers used fMRI and EEG to observe brain activity of an experienced meditator as he entered jhana, a meditative state of ecstatic joy. They were able to see how he voluntarily stimulated his dopamine/opioid reward system using only his own internal mental processes. (If this sounds crazy to you, I promise it’s not. While I’ve been practicing mindful movement for many years, I am not an experienced meditator like the test subject, and I am able to do what is described in this study, albeit to a less intense degree. I find it very effective for reducing stress and boosting happiness. I think it can and should be pursued by beginning meditators and certainly by Parkinson’s patients.)
How Parkinson’s patients report reducing or eliminating their motor symptoms
The first story I heard of someone fully recovering from Parkinson’s disease was that of Howard Shifke. He describes how he recovered in his book Fighting Parkinson’s…and Winning—an inspiring book that I recommend to all.
In September 2009, while working at his computer, Howard Shifke felt his entire body shaking on the inside. Howard had watched his mother suffer from Parkinson’s for 24 years, and he immediately went into a state of denial that he could possibly have the same condition. But he began to realize that symptoms had been gradually coming on for some time: hunched posture, worsening balance, a shuffling gait, muscle pain and stiffness, and fatigue, among others.
Six weeks later he visited a neurologist and got a formal diagnosis of Parkinson’s disease. Howard went into another state of denial—denial that his condition was incurable. He chose not to take any Parkinson’s medications due to the side effects, and immediately created a plan to recover from Parkinson’s drug-free. His approach included daily practice of meditation, medical Qigong, and switching to a vegetarian diet.
As the months went on, Howard found that his adrenaline-driven, judgmental, critical mind was holding him back from making progress. As he began to shift his habitual thought patterns into ones of acceptance, forgiveness, compassion, and love, he began to feel noticeable improvements in his symptoms.
On June 9th, 2010, nine months after Howard noticed his internal tremors, his symptoms suddenly decreased by 50%. Three days later, he awoke to find his symptoms completely gone. Ten years later, he is still symptom-free.
Howard now coaches people with Parkinson’s to help them reduce their symptoms and in some cases become symptom-free. You can read the stories of Tony C., Helen Gill, Marie, Pratima, and Betty M.—all of whom were diagnosed with Parkinson’s and used Howard’s Parkinson’s Recipe for Recovery® to become symptom-free. You can reach Howard through his website: https://www.fightingparkinsonsdrugfree.com
When I first read how Howard’s symptoms went away quite suddenly, I wondered what the scientific explanation could be. As I learned more about the progression of Parkinson’s and how motor symptoms appear at a critical threshold of dopamine production/neuron loss (estimated to be between 30% and 60%), it made sense. When people are able to boost their dopamine levels/number of neurons back up to that critical threshold, it is possible that motor symptoms may disappear just as suddenly as they started.
In 2016, doctors published a case report of a 78-year-old man’s remission of Parkinson’s symptoms, and cited meditation as a probable factor in his improvement:
“We present the case of a 78-year-old male who, 16 years ago, was diagnosed with Parkinson’s disease (PD) by a neurologist. He initially presented with left-hand tremor, stooped posture, shuffling gait, and frequent falls, which eventually progressed to bilateral motor symptoms after 3 years. Since 2012, his symptoms and signs have almost completely remitted, and he has been off all pharmacotherapy for that time. The accuracy of the initial PD diagnosis is supported by an appropriate clinical presentation, history of positive response to Sinemet, and an abnormal SPECT DaT scan; thus this case suggests the possibility of remission of symptoms in some patients. We propose that the patient’s long history of meditation practice may have been one contributing factor of this improvement as meditation has been shown to release dopamine in the striatum.”
In his book The Brain’s Way of Healing, Norman Doidge describes how John Pepper consciously retrained his movements to overcome his foot drag and tremor. John was diagnosed with Parkinson’s more than 20 years ago, but first started getting symptoms almost 50 years ago. He decided to begin his recovery in 1998.
John first focused on his walking, gradually training himself to fully support his weight and use both sides equally as he walked. It took him a year to internalize all of the changes and begin to walk normally. Then he decided to take conscious control of his tremor. Now, you would never know by looking at him that he has Parkinson’s. He doesn’t have a shuffling gait, he swings his arms when he walks, and has no visible tremor. He does not appear rigid, is able to initiate new movements fairly quickly, and has a good sense of balance. John has been off Parkinson’s medications for nine years.
In Pioneers of Recovery by Dr. Robert Rodgers, Bianca Mollé describes how she completely eliminated her motor symptoms using Qigong. When Bianca first began practicing Qigong, she immediately felt “one layer of pain strip away…there were many layers beneath that so it was a gradual process.” Soon she started forgetting to take her medication, because she wasn’t feeling tight and stiff. Her tremor was the last symptom to go away.
One year after Bianca began practicing Qigong, her neurologist declared her to be symptom-free. Two years later, the neurologist declared her to be Parkinson’s-free. Personally, she feels that she is still healing; she feels better and stronger every day. Bianca now offers online coaching for Parkinson’s patients and others with chronic illnesses, and you can watch her YouTube video about how she recovered.
Also in Pioneers of Recovery, Daniel Loney tells his story of how he has almost completely eliminated his motor symptoms using Tai Chi. When he was first diagnosed, he became very depressed, believing that there was no hope. His mother passed away shortly after his diagnosis, and he spent the next year mourning both her and his own life. Daniel’s condition went downhill rapidly; he almost quit practicing Tai Chi altogether, and he began to suffer from confusion, anxiety, and panic attacks.
Daniel began exploring alternative treatments, including Ayurvedic medicine, yoga, massage, acupuncture, and Chinese herbology. He felt that they all helped to some extent. Finally, he realized that in his Tai Chi practice he had everything he needed to heal himself.
Daniel left his stressful job and focused entirely on his recovery, putting an extreme emphasis on Tai Chi. His depression and mental symptoms went away almost immediately. As he regained strength, his tremor disappeared almost completely. He now feels very relaxed in his body; his stability, coordination, and strength have all improved; and he no longer stoops when he walks. It took about six months to a year for him to relieve his motor symptoms. Daniel now teaches Tai Chi for people with Parkinson’s in Israel. You can reach him through his website: https://www.taichiparkinsons.com
Parkinson’s patient Gord Summer has found vigorous exercise to be an essential part of his recovery. As he describes in Pioneers of Recovery:
”You start to get exhausted. Then you tap into your tenacity…I am always richly rewarded after pushing myself to the point of exhaustion. How? I walk the next day as if I were a young lad. Everything functions better…It makes stiffness take a backseat. It leaves Parkinson’s easily behind for an entire day. This has certainly been my personal experience.”
In Parkinson’s disease, motor symptoms are the external, observable signs of the changes that have occurred in the brain. When people are able to make lasting improvements in their motor symptoms, it is evidence of real changes they have made in their brains. Their stories are exciting and inspiring, and hopefully indicative of future changes in the approach to treating Parkinson’s disease.
Sheryl Marks Brown was diagnosed with a fast-progressing form of Parkinson’s in the summer of 2021. Tremors that normally take years to progress through the body took only five months to affect her entire body. Having cured herself in the 90’s from two autoimmune diseases, lupus and rheumatoid arthritis, Sheryl decided to try again to heal herself. She writes a blog to document the process, not knowing how it will end. You can read her blog and keep up with her inspiring journey of self-healing on her website, healingparkinsons.com.
In addition to mindfulness and mindful movement, there is a commonality among people who reduce or eliminate their motor symptoms: they believe they can get better. Expectation induces neurochemical changes in the brain. Researchers at the University of British Columbia demonstrated that simply believing their symptoms would improve triggered the release of dopamine in Parkinson’s patients. Further research replicated the results. Positron emission tomography (PET) scans show that belief stimulates the release of dopamine in the striatum of Parkinson’s patients, causing complete elimination of motor symptoms for a period of time. Dr. Joe Dispenza describes one such study in this video.
Likewise, studies like this, this, and this have shown that motor function in Parkinson’s patients improves when they receive placebo treatment or have the expectation that their symptoms will improve.
On the flip side, believing you will not improve deactivates the dopaminergic system. So in addition to stimulating neurogenesis and boosting dopamine with exercise and stress reduction, believing that you can improve is essential in your recovery.
Do you have Parkinson’s disease, and do you want to improve your motor symptoms? I recommend talking to Howard or Bianca, both of whom have eliminated their motor symptoms and now offer coaching. You may decide to follow their paths or create your own personalized prescription. And always remember: Your health is in your hands, and the more strongly you believe that you can improve and recover, the more likely it is that you will.
Recommended reading:
The Pain Relief Secret: How to Retrain Your Nervous System, Heal Your Body, and Overcome Chronic Pain by Sarah Warren, CSE
Somatics: Reawakening the Mind’s Control of Movement, Flexibility and Health by Thomas Hanna

