Doctors Call for an End to the Parkinson’s Pandemic
Parkinson’s disease is not new—descriptions of the condition can be found in ancient Chinese medical texts from 425 BC and Indian medical texts from 600 BC. There have been references to Parkinson’s symptoms in documents from Greek, Roman, and other civilizations over the past two thousand years. However, it’s assumed that the condition used to be relatively rare, and it did not have a universally recognized name until recently.
In the early 19th century, the Industrial Revolution was in full swing in Europe and the United States. The air and water in London had become heavily polluted with new chemicals and toxins, leading to the term “London fog.” Dr. James Parkinson observed six men on the streets of London who exhibited what we know today to be the classic symptoms of Parkinson’s disease: tremors, stooped posture, shuffling gait, and a tendency to fall. He described these case studies in 1817 in “An Essay on the Shaking Palsy.” By the end of the 19th century, Parkinson’s disease was widely recognized by the medical community, but the cause was still unknown. It was only in hindsight that London’s pollution provided a clue.
In the 1950s, Swedish pharmacologist Dr. Arvid Carlsson identified the neurotransmitter dopamine, and in laboratory experiments with rabbits he discovered that dopamine plays an essential role in the brain’s control of movement. He also discovered that giving the rabbits levodopa (L-dopa), which is converted into dopamine in the brain, allowed them to regain normal movement.
Humans naturally synthesize levodopa from the amino acid L-tyrosine. When L-dopa was administered as a drug to Parkinson’s patients, the effects were astounding. L-dopa is still the most effective drug for managing the symptoms of Parkinson’s disease. Unfortunately, L-dopa is not a cure.
It wasn’t until 1982, when doctors observed a case of Parkinson’s that occurred suddenly as a result of taking a synthetic version of heroin, that scientists figured out that a chemical could kill dopamine-producing neurons and cause the condition. Soon they discovered that commonly used pesticides had the same effect.
Pesticides and other toxins are clearly one of the causes of Parkinson’s disease; the prevalence of Parkinson’s began increasing during the Industrial Revolution, and spiked again when synthetic pesticides were introduced in the mid-20th century. The least industrialized countries in the world have the lowest rates of Parkinson’s, while countries like China that are rapidly becoming more industrialized and polluted have the highest rates of increase.
In March 2020, four neurologists and neuroscientists (Drs. Ray Dorsey, Todd Sherer, Michael S. Okun, and Bastiaan R. Bloem) released the book Ending Parkinson’s Disease: A Prescription for Action. The book is heavily focused on how widespread use of pesticides and other neurotoxic chemicals have made Parkinson’s disease the world’s fastest growing neurological disorder.
Approximately 0.3% of the general population, and 1% of people over age 60, have Parkinson’s disease. These percentages equate to 6.2 million people as of 2015, and The Global Burden of Disease Study estimates that there will be at least 12.9 million people with Parkinson’s globally by 2040.
The authors of Ending Parkinson’s Disease call Parkinson’s a man-made pandemic; instead of being caused by a virus or bacteria, it’s caused by “urbanization, population aging, globalization, and widespread availability of unhealthy products.” Their “Prescription for Action” has four steps:
- Prevent: Ban the use of neurotoxic chemicals and make lifestyle changes
- Advocate: Push for increased research funding
- Care: Expand and improve diagnosis and medical care
- Treat: Participate in research studies
Despite the overwhelming evidence for the role of man-made neurotoxic chemicals in Parkinson’s disease, it’s clear that these chemicals are not the only cause. The authors also describe other factors that contribute to Parkinson’s: genetics, traumatic head injuries, lack of exercise, diet, and gut dysbiosis. They don’t present research on the role of chronic stress, the evidence for which is mounting, but I’ve included it in this post. The authors write:
“Two hundred years after Parkinson’s essay, researchers have identified many causes of Parkinson’s, but more of them remain to be found. Like cancer, Parkinson’s is not one disease but rather a collection of many with different contributing factors.”
It is widely agreed in the scientific community that Parkinson’s is most often the result of a complex interplay between genetic and environmental factors—this is what has made it so difficult to define, treat, and prevent. Having one risk factor is often not enough to trigger the condition, but having two or more increases the odds substantially. It can be hard to wrap our minds around the fact that such a variety of factors can all result in the same, or similar, pathology. But this is true for many chronic conditions including cancer, Alzheimer’s disease, and Parkinson’s disease.
The authors include an insightful quote from Parkinson’s expert Dr. William Weiner: “There is no single Parkinson disease…there never has been.”
Dr. Weiner felt that “the misunderstanding of Parkinson disease may be hindering clinical research trials.” He proposed use of the term “Parkinson diseases” and suggested that different versions of the disease have different causes, symptoms, rates of progression, and treatments.
In this post I’ll summarize some of the most important points from Ending Parkinson’s Disease, and discuss how genetics, toxins, head trauma, lack of exercise, diet and gut health, and stress all contribute to Parkinson’s. Where I have not linked to research, the studies and statistics are referenced and described in Ending Parkinson’s Disease. Be sure to read next week’s post on how people use exercise and stress reduction to boost dopamine, enhance the natural process of neurogenesis, reduce the motor symptoms of Parkinson’s, and in some cases eliminate motor symptoms completely.
What is Parkinson’s disease?
Parkinson’s disease is a degenerative neurological condition in which dopaminergic (dopamine-producing) neurons in a part of the brain called the substantia nigra die off. When there is not enough dopamine in the nigrostriatal pathway (shown in pink in the diagram below), motor symptoms occur. Other parts of the brain suffer neurodegeneration as well, causing some of the non-motor symptoms of Parkinson’s.
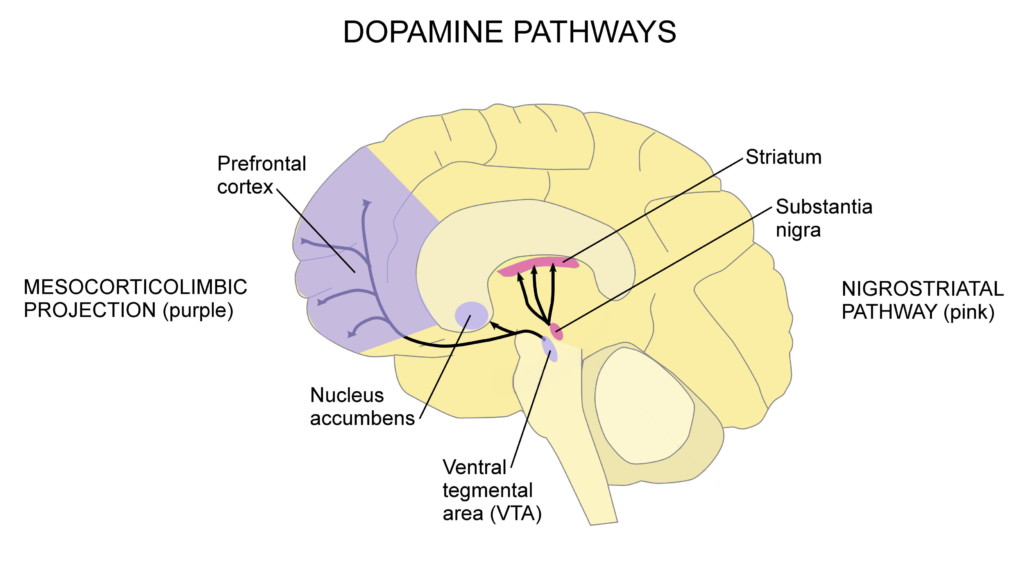
Parkinson’s disease typically gets diagnosed when motor symptoms appear. The average age of diagnosis is age 60. When Parkinson’s is diagnosed after age 50, it is called late-onset; diagnosis before age 50 is called early-onset or young-onset. Diagnosis before age 20 is referred to as juvenile-onset. The younger someone is at age of diagnosis, the more likely it is that genetics play a role in their disease.
Late-onset Parkinson’s disease is the most common form, and risk increases with age. The longer we live, the more time there is for neuron loss to occur, and more the more chance there is for Parkinson’s to develop. As the world population continues to age, we’ll see rising rates of the disease unless major changes are made in pesticide regulations and lifestyle.
A defining aspect of Parkinson’s disease is Lewy bodies, which are misfolded clumps of the protein alpha-synuclein. Lewy bodies develop in the substantia nigra and other brain areas, as well as the enteric nervous system (ENS; neurons that control the gastrointestinal tract), and cause neuron death. Lewy bodies are present in almost all cases of Parkinson’s disease. Typically when symptoms are present without Lewy bodies, it is referred to as parkinsonism. Parkinsonism can be the result of other neurological diseases, medications, and infections.
Symptoms can begin 20+ years before diagnosis—Dr. Heiko Braak explains why
The most common motor symptoms of Parkinson’s disease include resting tremor (shaking or trembling when at rest), rigidity or stiffness, slow movement (bradykinesia) or the inability to move, a shuffling gait, postural instability, and impaired balance and coordination. Other motor symptoms include:
- Dystonia (painful muscle cramps)
- Stooped posture
- Impaired fine motor dexterity and motor coordination
- Impaired gross motor coordination
- Decreased arm swing
- Akathisia (tendency to keep moving)
- Speech problems
- Difficulty swallowing
- Sexual dysfunction
Non-motor symptoms include disturbed sleep, anxiety, depression, pain, cognition difficulties, visual hallucinations, and dementia.
Unfortunately, motor symptoms typically appear years after the disease process has begun—often 20 or more years after. It’s estimated that motor symptoms appear when approximately 30% to 60% of dopaminergic neurons in the substantia nigra are lost. Studies show varied results when it comes to the actual percentage of neuron loss necessary to produce motor symptoms, and it’s safe to say that the exact percentage is different from person to person. Regardless of the percentage, it is understood that when dopamine levels decrease to a certain critical threshold, tremor or other motor symptoms may be suddenly felt (I’ll discuss this in more detail in next week’s post). For many people, this is the first noticeable sign of the disease.
Once diagnosed, people are often able to recognize symptoms that occurred years earlier. Decades before motor symptoms begin, people may experience loss of sense of smell and constipation. These occur because the disease attacks the medulla (a part of the brain stem), the olfactory bulb (brain area responsible for sense of smell), and the enteric nervous system (ENS; neurons that control the gastrointestinal tract) early on.
Next, depression and rapid eye movement sleep behavior disorder may occur as the disease progresses to the pontine tegmentum (a part of the brain stem) and other areas. When the disease progresses to the substantia nigra and does sufficient damage, motor symptoms occur. Dementia and hallucinations may occur in the latest stages of the disease, as neural damage spreads to the neocortex.
In 2003, Dr. Heiko Braak along with his late wife Dr. Eva Braak and a team of researchers clearly demonstrated this progression of Parkinson’s through the nervous system in autopsy studies. That same year, Braak proposed the hypothesis that Parkinson’s disease does not begin in the central nervous system; instead, the pathogens that cause the disease enter the body through the nose and gut. This is what happens when we inhale or ingest a toxin.
Braak’s hypothesis explains why the first symptoms of the disease are loss of sense of smell and constipation. Further research has backed up his theory, showing how Lewy bodies are found in the nerves of the nose and intestines first—before they are found in the central nervous system. The pathology of Parkinson’s spreads from nerve cell to nerve cell, gradually progressing upward. Scientists believe that the vagus nerve, which connects the brain stem and gut, is the likely route by which the disease travels to the brain. Since there is “no single Parkinson disease,” this may not be how all cases progress, but substantial research suggests that it is how many cases progress.
The role of genetics
Researchers estimate that between 10% and 15% of people with Parkinson’s disease have a genetic predisposition. Mutations in the LRRK2, PARK7, PINK1, PRKN, SNCA, GBA, and UCHL1 genes, or in other genes that have not yet been identified, can increase risk of developing the condition.
Some gene mutations affect the way that proteins are broken down in the nervous system, and when proteins accumulate, neuron death may occur. Mutation of the SNCA gene causes alpha-synuclein to misfold and form Lewy bodies. Other gene mutations may affect the function of mitochondra (energy-producing structures within cells), leading to an accumulation of free radicals and resulting in death of dopaminergic neurons.
Most of the time, genetic mutations alone are not enough to cause Parkinson’s disease—other factors must be present also. For example, when the SNCA mutation is present, exposure to “safe” levels of certain pesticides can lead to disease. And a study of more than 17,000 twin brothers showed that for typical late-onset Parkinson’s disease, environmental factors are more important than genetics.
The role of neurotoxic chemicals
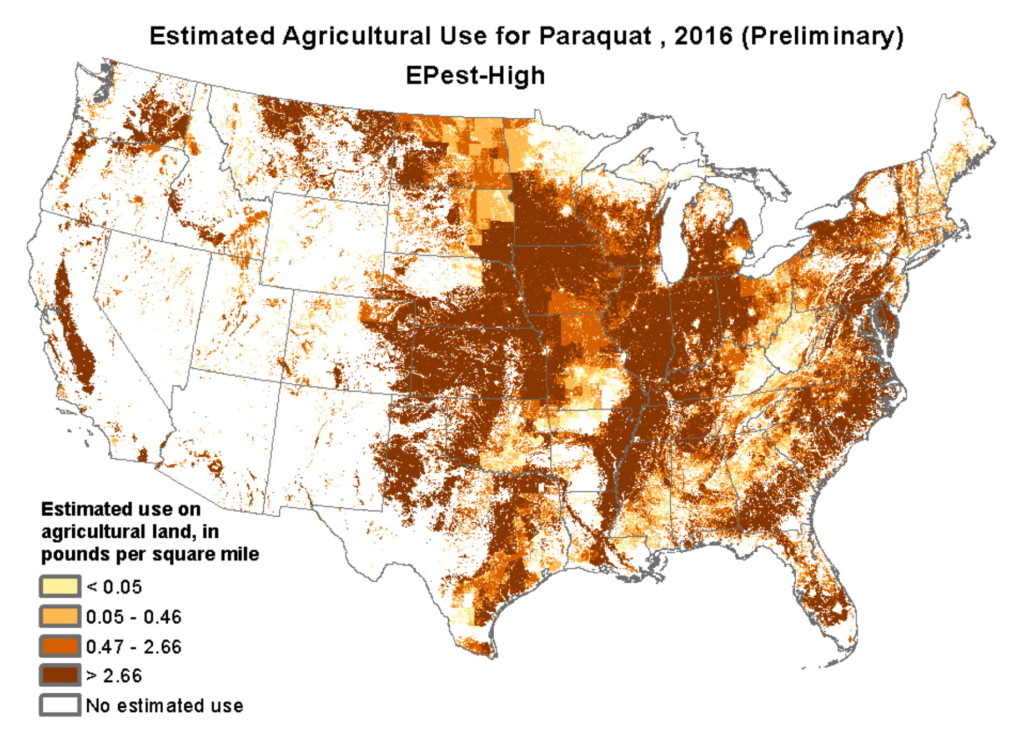
Exposure to pesticides and other neurotoxic chemicals significantly increases the risk of Parkinson’s disease. The US Environmental Protection Agency (EPA) is lagging behind when it comes to banning chemicals that have been shown to cause Parkinson’s and other diseases. For example, 32 countries, including China, have banned paraquat, a pesticide that increases the risk of Parkinson’s by 150%. The Netherlands banned paraquat, trichloroethylene (a solvent), and other pesticides linked to Parkinson’s and their rates of Parkinson’s declined sharply. The United States needs to do the same, but financial profit is clearly taking precedence over public health and safety.
Instead of being banned, the use of paraquat on US farmland has doubled over the past decade. Paraquat is shown in laboratory studies to kill dopaminergic neurons, and it can also cause blindness and internal bleeding. Swallowing a teaspoonful is fatal, and because it is so readily available, people use it to commit suicide. In agriculture, paraquat is primarily used in the farming of corn, soybeans, wheat, cotton, and grapes. The map below shows the estimated usage of paraquat in the US.
The use of paraquat and other pesticides are the reason that agricultural areas have the highest rates of Parkinson’s disease. Farmers have 170% greater risk than non-farmers of developing Parkinson’s, and the longer they are exposed to pesticides, the greater their risk. And simply living in agricultural areas increases risk of Parkinson’s because the air, groundwater, and well water can be polluted with toxic pesticides.
The industrial solvent trichloroethylene (TCE) also contributes to Parkinson’s, as well as cancer. People exposed to TCE at work are six times more likely to develop Parkinson’s. Until 1977, TCE was used as a surgical anesthetic, and today it is still used to degrease metal, in dry cleaning, and in household products like paint removers, stain removers, glue, carpet cleaners, and gun cleaners. Even though TCE has been known to be toxic since the 1930s, the United States uses about 250 million pounds of the chemical every year.
In rat experiments, TCE kills dopaminergic neurons in the substantia nigra. One study found that 68% of workers at a small industrial plant in Kentucky had symptoms of Parkinson’s, and those who had the most exposure to TCE were most likely to have symptoms. TCE can be breathed in through polluted air, ingested through contaminated water, and absorbed through the skin. The EPA has stated that TCE is “carcinogenic to humans by all routes of exposure” and that chronic exposure is damaging to the brain and nervous system.
Frighteningly, up to 30% of the US drinking water supply is contaminated with TCE. Almost half of Superfund sites (designated toxic waste dumping sites) are contaminated with TCE, and local residents have higher rates of Parkinson’s disease and cancer.
There are other chemicals linked to Parkinson’s disease as well, including Agent Orange, an herbicide used in the Vietnam War to kill vegetation so that aircraft fighters could see the enemy on the ground below. The dry cleaning solvent perchloroethylene (PCE), the insecticide DDT, and the pesticide heptachlor are others. There may still be more that have not been identified.
The use of toxic chemicals in farming, industry, and certain products is one of the reasons why men are diagnosed with Parkinson’s 40% more often than women. In the US, 75% of farmers are men, along with 80% of metal and plastic laborers, 90% of chemical workers, 91% of painters, 96% of welders, and 97% of pest control workers.
Pesticides and other toxic chemicals kill dopaminergic neurons in a number of ways. They can damage the cytoskeleton, mitochondra, or myelin sheath (protective coating); cause oxidative stress or an overload of calcium; interfere with neurotransmission; or inhibit ALDH enzymes that detoxify the dopamine metabolite DOPAL.
While exposure to certain toxic chemicals increases the risk of developing Parkinson’s, exposure alone usually does not mean that a person will definitely get the disease. Just as at least 75% of heavy smokers will not get lung cancer, most people who are exposed to pesticides and other neurotoxic chemicals will not get Parkinson’s. It’s when pesticide exposure and other risk factors, like genetics and the lifestyle factors I’ll describe next, come together that risk increases dramatically.
The role of head trauma
In recent years, the long term negative effects of repetitive head injuries, like those suffered by professional football players, has become widely recognized. Football players are more likely to suffer from Alzheimer’s disease, amyotrophic lateral sclerosis (ALS; Lou Gehrig’s disease), chronic traumatic encephalopathy (CTE), and Parkinson’s disease. In a 2017 study of 111 former NFL players, two-thirds of them had parkinsonian symptoms including tremor, slow movement, difficulty walking, and a tendency to fall.
Traumatic brain injury is also common among military veterans, and athletes like boxers and ice hockey, soccer, and lacrosse players. A single head injury resulting in loss of consciousness triples the risk of Parkinson’s disease, and repeated injuries raise the risk further.
Head trauma can trigger proteins to clump together, forming the Lewy bodies of Parkinson’s and the amyloid plaques of Alzheimer’s. These protein clumps can cause cell membranes to burst, leading to neuron death. Scientists suggest that more research is needed to investigate whether the form of Parkinson’s disease linked to head trauma is the same as that which is present in people who have no history of head trauma.
The role of lack of exercise
Given that movement is affected in Parkinson’s disease, it makes sense that regular exercise would help to prevent it as well as slow the progression. In a study of more than 7,300 male veterans, those with the lowest level of physical fitness had a 316% higher risk of developing Parkinson’s than those with the highest level of physical fitness.
Like other research involving genetics, pesticides, and head injury, this study found significant increased risk of developing Parkinson’s when multiple risk factors were involved. The researchers looked at age, physical fitness, and smoking (which surprisingly, decreases risk). They found that people with two of the risk factors were 3.7 times as likely to develop Parkinson’s, and people with all three of the risk factors were 7.8 times as likely.
Reduced physical activity is likely an early symptom of Parkinson’s disease; as movement becomes more difficult, people are less likely to move. Neuroscientists at the University of Texas in Austin did an interesting study to explore whether or not a reduction in physical activity also speeds up the degeneration of dopaminergic neurons. By restricting the movement of rats, they were able to show that the restriction of movement led to severe and chronic loss of dopaminergic neurons and impaired motor function. Neurons that aren’t used tend to wither away and die—this is called neural pruning. When it comes to movement, the principle of “use it or lose it” applies not only to our muscles, but to the health of our brain as well.
On the flip side, when we use certain parts of our brain a lot, it stimulates neuroplasticity and neurogenesis in those areas. And exercise stimulates neuroplasticity and neurogenesis throughout the brain by triggering the release of neurotrophic factors, which encourage synaptic plasticity, promote the initial growth and development of neurons, support neuronal survival, and even regrow damaged neurons. Not only does regular exercise help to prevent Parkinson’s, but it also improves motor symptoms and slows progression of the disease. Research shows how exercise prevents the loss of, protects, and actually restores dopaminergic neurons in Parkinson’s disease; I’ll discuss this in detail in next week’s post.
The role of diet and gut dysbiosis
There are a number of ways in which diet and gut health contribute to Parkinson’s disease. I’ll start with some of the overwhelming evidence of the benefits of a plant-based diet and the harmful effects of a diet high in animal protein.
A 2007 study of 130,000 people found that a Mediterranean-style diet (high in fruit, vegetables, and fish) was associated with a 22% reduced risk of Parkinson’s when compared with the Western-style diet (high in red meat, processed and fried food, refined grains, sugar, and high-fat dairy products). The authors of Ending Parkinson’s Disease suggest that one way a Mediterranean-style diet may be protective is that it’s high in antioxidants, which may reduce the development of Lewy bodies. Antioxidants also prevent damage to mitochondria, which are damaged in Parkinson’s disease.
Along those same lines, research shows that high consumption of dairy products is associated with an 80% increased risk of Parkinson’s in men and a 30% increased risk in women.
And in 2011, a study of Parkinson’s patients showed significant improvement of motor symptoms after just four weeks on a plant-based diet, when compared to a control group on an omnivorous diet. The explanation for the quick results may be explained by the connection between animal protein and levodopa.
A fascinating study showed how consumption of animal protein elevates levels of large neutral amino acids (LNAAs), which compete with levodopa for transport across the blood-brain barrier. On regular and high-protein diets, patients had parkinsonian motor symptoms despite having elevated levels of levodopa in their blood from medication. When patients were put on a low-protein diet, their LNAA levels dropped, and they were relieved of their motor symptoms.
Other research shows that levodopa levels and motor symptoms are both improved on a high-fiber diet. If you have three minutes, watch this video by Dr. Michael Greger about diet and Parkinson’s disease.
Related to diet and other factors, gut dysbiosis is often present in Parkinson’s disease. Gut dysbiosis is an imbalance in the gut microbiome, the collection of microorganisms, bacteria, viruses, protozoa, and fungi in the digestive tract.
Gut dysbiosis is associated with many health issues, from neurodegenerative diseases to digestive problems, autoimmune conditions, allergies, anxiety and depression, skin conditions, obesity, and diabetes. A thorough 2019 review discusses the role of gut dysbiosis, inflammation, and leaky gut in Parkinson’s disease, and the potential role of probiotics and fecal transplantation in treatment.
A 2016 study showed that Parkinson’s symptoms are actually transmissible through fecal matter. Researchers gave mice fecal material from healthy human subjects, and their motor function was unchanged. But when the mice were given fecal material from people with Parkinson’s, their motor function worsened. Researchers suggest the possibility that fecal transplants may at some point be used to treat Parkinson’s disease.
While fecal transplants are becoming more mainstream, and rightly so, they may not always be necessary. Consuming a whole-food, plant-based diet, along with pre- and probiotics, as well as avoiding antibiotics, can go a long way toward balancing out your gut bacteria. Stress, lack of exercise, and insufficient sleep also have negative effects on the gut microbiome.
Also related to diet and other factors, constipation is a very common early symptom of Parkinson’s. Men with constipation are 2.7 times more likely to develop Parkinson’s than men without. As I mentioned earlier, it’s believed that constipation occurs early on in the disease process because of the development of Lewy bodies in the enteric nervous system. It has also been suggested that constipation can contribute to gut dysbiosis because food stays in the digestive tract longer than it should. Whether or not you have Parkinson’s, the simplest ways to get things moving are consuming lots of fruit, vegetables, whole grains, and legumes; staying hydrated; and exercising regularly.
The role of chronic stress
Chronic stress increases the risk of virtually all chronic diseases, including cancer, heart disease, autoimmune conditions, and neurodegenerative diseases. Stress plays a role in Parkinson’s in three ways:
1. Depression is an early symptom of Parkinson’s, and researchers suggest that chronic stress preceding the onset of motor symptoms may be an early symptom as well.
2. Having Parkinson’s disease is very stressful, as it limits one’s function and has a poor prognosis.
3. Chronic stress contributes to the onset and worsening of Parkinson’s disease by increasing neuroinflammation and neurodegeneration.
The third way is becoming more widely researched, as stress is finally being recognized as a risk factor for Parkinson’s. A 2017 study found that mice subjected to chronic stress experience loss of dopaminergic neurons in the substantia nigra—the same neurons lost in humans with Parkinson’s. And a 2014 study found that the inflammatory response in stressed rats led to a higher rate of death of dopaminergic neurons in the substantia nigra. This confirmed previous research showing that stress and elevated glucocorticoid levels lead to death of these dopaminergic neurons.
The stress response is regulated by the hypothalamic-pituitary-adrenal axis (HPA axis), the functional connection between the hypothalamus, pituitary gland, and adrenal glands. When we perceive stress, our hypothalamus releases corticotropin-releasing hormone (CRH), which in turn stimulates our pituitary gland to release adrenocorticotrophic hormone (ACTH). ACTH then stimulates our adrenal cortex to release cortisol and other corticoid hormones, which act on nearly every tissue in our body. The HPA axis is the mechanism by which stress and emotions affect our health. Chronic stress can cause dysregulation of the neuroendocrine and immune systems, leading to neuroinflammation, oxidative stress, and loss of dopaminergic neurons.
A study of 330 people showed the link between stress and risk of Parkinson’s; risk increased significantly with the number of stressful life events that people had experienced. A population-based study of over 2.5 million residents of Sweden found that job-related stress was associated with an increased risk of Parkinson’s disease. And stress-related personality traits, like being anxious, pessimistic, and neurotic, are associated with an increased risk of Parkinson’s.
Stress level also predicts worse disease progression. Acute stress worsens motor symptoms of Parkinson’s, including bradykinesia, freezing, and tremor. In a study of 4,155 Parkinson’s patients, stress predicted mortality and worsening mobility.
Moving toward improvement and recovery
While there is no pharmaceutical or surgical cure for Parkinson’s disease, there is hope. Scientists are optimistic about non-invasive treatment approaches that stimulate neurogenesis and mobilize endogenous neural stem cells—those that are naturally produced in the brain—to survive, migrate, and differentiate into dopaminergic neurons in the substantia nigra. But you don’t have to wait for these treatments to be approved.
Researchers agree that exercise—especially vigorous exercise—should be prescribed to Parkinson’s patients as a component of treatment. They note that there is currently no pharmacological treatment that can modify or slow the disease or protect dopaminergic neurons the way that exercise does. For Parkinson’s patients, exercise does what medication can’t.
Most exciting of all, there are Parkinson’s patients who report significantly improving their motor symptoms and in some cases, eliminating them completely. For most of them, some form of mindfulness or stress reduction played a large role in their recovery. I’ll tell their stories, discuss neurogenesis as it applies to Parkinson’s, and explain how exercise and stress reduction can heal the damage done to the brain by Parkinson’s disease in next week’s post.
Recommended reading:
The Pain Relief Secret: How to Retrain Your Nervous System, Heal Your Body, and Overcome Chronic Pain by Sarah Warren, CSE
Somatics: Reawakening the Mind’s Control of Movement, Flexibility and Health by Thomas Hanna