What is Chronic Inflammation, and Why Is It Killing Us?
If it seems like you hear a lot about inflammation, you’re right. It’s become the latest buzzword in the health world due to research showing the link between inflammation and many chronic conditions including cancer, heart disease, diabetes, dementia, and depression. Ever since these “diseases of civilization” began to take the place of infectious diseases, researchers have been searching for an equivalent to germ theory—the nineteenth century discovery that led to groundbreaking advances including antibiotics, immunization, and pasteurization.
During the 1990s and early 2000s, research that demonstrated the role of inflammation in diseases such as cancer and diabetes began to accumulate. As the body of evidence continues to grow, the medical community may now be converging on a unified theory of chronic disease based on the concept that chronic, low-grade systemic inflammation is an underlying and maintaining factor in many chronic lifestyle-related and toxin-induced conditions.
In this post, I’ll discuss what inflammation is, and how it causes and contributes to cancer, atherosclerosis, and type 2 diabetes.
What is inflammation?
Inflammation is part of our body’s natural, protective response to a dangerous invader (like a bacteria, virus, or toxin) or damage to our body’s own cells.
Our immune system does not discriminate between pathogenic or toxic invaders and physical trauma. Proteins called pattern recognition receptors detect both dangerous invaders and physical cell damage, and our immune system responds in the same way to both types of attacks.
When these threats to our survival are perceived, our immune system goes into high gear in its effort to remove the harmful stimuli and begin the healing process. Within moments, blood vessels dilate and increased blood flow makes the area of injury or infection feel warm and appear red. Capillaries become permeable, allowing white blood cells to move from the bloodstream to the injured area. This causes swelling, which helps to isolate the invaders or damaged cells from the rest of the body.
The functions of the inflammatory response are to:
1. Isolate the dangerous invader or damaged cells
2. Clear away dead cells and other damaging substances
3. Begin the repair process
Whenever I hear the word inflammation, I immediately translate that in my head to “immune system response.” This reminds me why it’s happening: because the body is in defense mode. If inflammation is occurring, that means that the cells in our body are detecting something that might be harmful to us.
There are two different types of inflammation:
Localized inflammation occurs at the site of an injury or infection. A sprained ankle that is swollen and painful, or an infected cut that is red and swollen, are both examples of localized inflammation.
Systemic inflammation occurs throughout the body. This type of inflammation can be triggered by external sources including viral and bacterial infections, allergens or toxins in the food we eat and in our environment, smoking, and alcohol consumption. It can also be triggered by internal states including stress, obesity, autoimmune conditions, and genetic variations. (I’ll discuss why all of these things cause inflammation in an upcoming post!)
When localized or systemic inflammation is acute, or lasting just a few days, it is a good thing. We rely on this critical function of our immune system to heal our injuries and keep us protected from dangerous invaders.
Unfortunately, sometimes inflammation becomes chronic, lasting months or years. Inflammation can become chronic when the cause of inflammation continually occurs, when acute inflammation doesn’t resolve the situation, or when an autoimmune condition sets in.

“From the wings to center stage: How inflammation triggers a multitude of diseases – Longwood Seminar.” Harvard Medical School. Streamed live on April 18th, 2017.
Our immune system evolved to expertly deal with short-term threats like injuries and infections. It did not evolve to deal with the never-ending barrage of threats that we now subject it to every day—like chronic stress, processed meat, and environmental toxins. As a result, chronic inflammation has many negative health effects and contributes to a wide range of health conditions (see chart below).
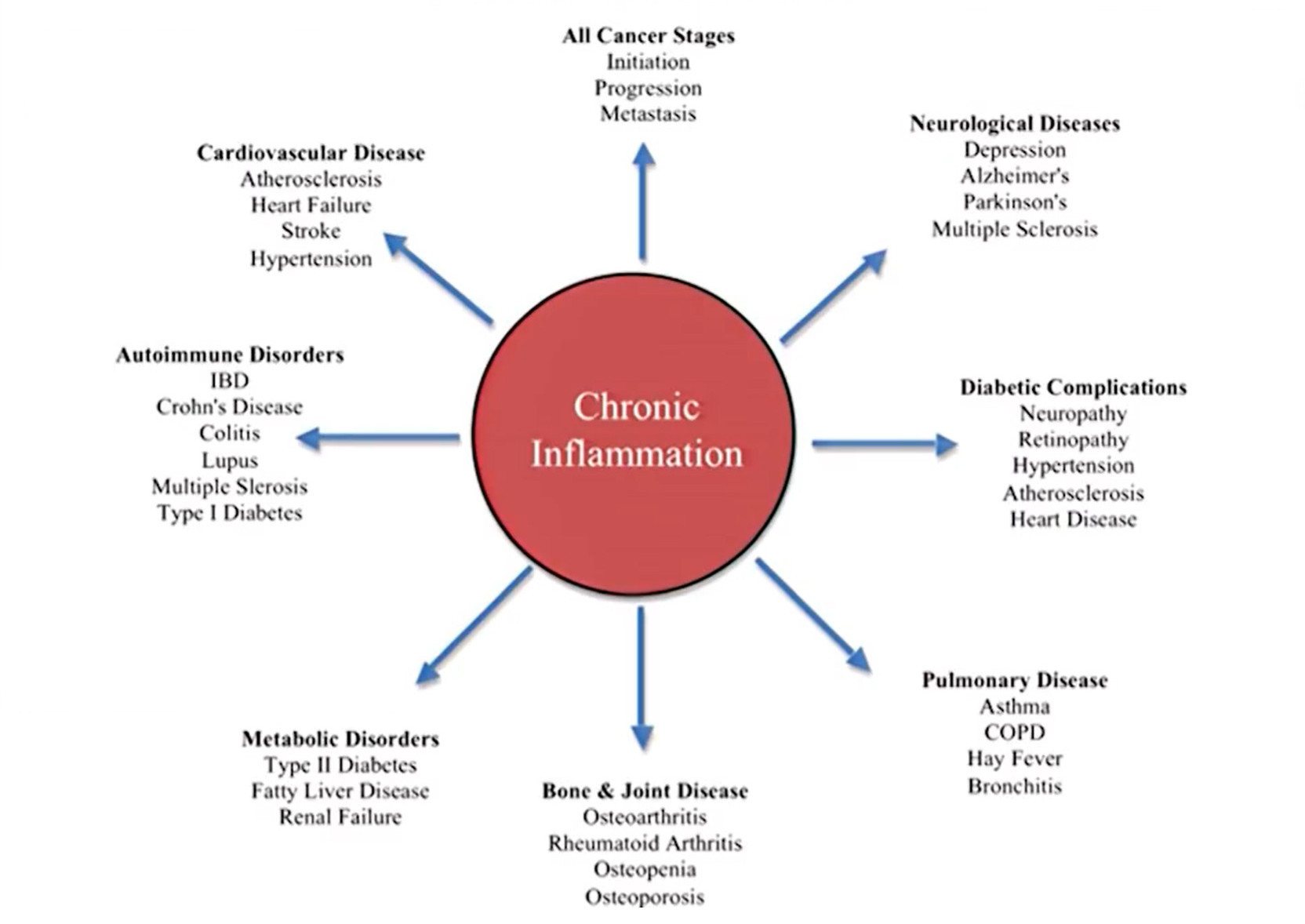
“From the wings to center stage: How inflammation triggers a multitude of diseases – Longwood Seminar.” Harvard Medical School. Streamed live on April 18th, 2017.
How chronic inflammation causes and contributes to cancer
Cancer cells start out as normal, healthy cells. They become cancerous when their DNA (genetic material) mutates, or is altered. Cancer can start in any cell in the body.
While some people are born with genetic mutations that lead to cancer, these account for a small percentage of cancer cases. Most people get cancer due to genetic mutations that occur after birth. There are many causes of these gene mutations, including smoking, radiation, viruses, cancer-causing chemicals (carcinogens), obesity, hormones, alcohol consumption, lack of exercise, and chronic inflammation. It is estimated that up to 20% of cancers are related to chronic infections, 30% result from tobacco smoking and inhaled pollutants, and 35% are related to dietary factors, including obesity.
The most common gene mutations that occur to create cancerous cells are ones that:
- Create rapid growth: A gene mutation can tell the cell to grow and divide more rapidly than normal; this creates many copies of this cell that all have the same mutation.
- Fail to stop cell growth: Normal cells have tumor suppressor genes that tell the cells when to stop growing, so that we don’t have too many of a certain type of cell. A mutation in a tumor suppressor gene allows cancer cells to keep growing and replicating.
- Alter DNA repair genes: Normal cells have DNA repair genes that repair errors in the cells’ DNA. A mutation in a DNA repair gene can allow errors to go uncorrected, leading the cell to become cancerous.
Now that we understand how and why normal cells become cancerous, let’s look at the two ways in which chronic systemic inflammation causes and contributes to cancer:
1. Chronic inflammation can cause the gene mutations in healthy cells that lead them to become cancerous. Research shows that gene mutations occur as a result of stress induced by the production of reactive oxygen and nitrogen species during the inflammatory process. Inflammation results in decreased expression of tumor suppressor genes and alteration of DNA repair pathways.
2. Cancer cells lure immune system cells into the tumor, resulting in tumor growth. The recruited immune system cells help to grow blood vessels within the tumor, contribute to cell growth within the tumor, and cause further gene mutations.
Now that the strong link between inflammation and cancer has been proven, research is showing how lifestyle modifications like a plant-based diet, exercise, and stopping smoking and alcohol consumption are effective in reducing inflammation and cancer risk. It has also been shown that anti-inflammatory drugs such as aspirin can reduce inflammation and cancer risk.
How chronic inflammation causes and contributes to atherosclerosis
Atherosclerosis is the buildup of plaques in arteries, also called hardening of the arteries. When these plaques rupture, they can cause blood clots, heart attacks, and strokes.
As you can see in the illustration below, plaques build up in the walls of arteries under the endothelium, the thin membrane that lines the inside of the arteries. The plaques are made up of fat, cholesterol, calcium, and other substances found in blood.

“From the wings to center stage: How inflammation triggers a multitude of diseases – Longwood Seminar.” Harvard Medical School. Streamed live on April 18th, 2017.
Why do these plaques build up? Cholesterol has long been blamed for atherosclerosis, but high cholesterol levels alone may not be enough to cause plaques to form. In this 2011 study, researchers genetically modified mice so that the mice could not synthesize histamine, a compound that our bodies produce during inflammation and in response to stress. Without histamine, the mice did not form endothelial cell plaques—even though their blood cholesterol levels were high as a result of being fed a high-cholesterol diet. These results suggest that the histamine produced during our natural immune or stress response plays a critical role in atherosclerosis.
When plaques have formed, immune system cells migrate to the plaques and actually eat the cholesterol. The immune system cells get bigger and turn into foam cells which stay in place. They create waste, contribute to plaque buildup, and increase the risk of plaque rupture. In the illustration below, you can see how leukocytes (immune system cells) make up a large part of atherosclerotic plaques.

”From the wings to center stage: How inflammation triggers a multitude of diseases – Longwood Seminar.” Harvard Medical School. Streamed live on April 18th, 2017.
Research shows that high levels of C-reactive protein (CRP) in the blood coincide with higher rates of atherosclerosis; CRP is a sign of inflammation occurring in the body. And as you might expect, the usual inflammation-causing lifestyle-related factors increase the risk of developing atherosclerosis: smoking, alcohol consumption, stress, obesity, diabetes, an unhealthy diet, and lack of exercise.
How chronic inflammation causes and contributes to type 2 diabetes
Diabetes occurs when the level of glucose (sugar) in the blood becomes too high. Blood glucose is our main form of energy, and it comes from the food we eat. Insulin, a hormone made by our pancreas, allows glucose to move from our blood into our muscle cells so that it can be used for energy.
In type 2 diabetes, the body does not use insulin well (insulin resistance) or does not make enough insulin. As a result, glucose stays in the blood and builds up, leading to high blood glucose levels. Type 2 diabetes can develop at any age, and the greatest risk factor is body weight: about 87.5% of people with type 2 diabetes are overweight or obese.
As Dr. Michael Greger shows in this video, people eating a high-fat diet have higher levels of blood glucose than people eating a high-carbohydrate diet. Fat in the bloodstream can build up inside muscle cells, blocking insulin from doing its job of letting glucose into the muscle cells; this is how excess fat contributes to insulin resistance.
So, what role does inflammation play in insulin resistance? Researchers discovered that in people with type 2 diabetes, levels of pro-inflammatory cytokines (signaling proteins in the immune system) inside fat tissue are elevated. Their conclusion was that obesity-induced immune response contributes to insulin resistance. In other words, having excess body fat triggers inflammation in our body, which leads to a buildup of cytokines in fatty tissue, which prevents insulin from doing its job.
A second way in which inflammation contributes to type 2 diabetes is that once insulin resistance has developed, the insulin resistance itself can cause further inflammation. This can result in a vicious cycle in which inflammation causes insulin resistance and insulin resistance causes inflammation, leading to a continuous worsening of the condition.
About 12.5% of people with type 2 diabetes are not overweight, but inflammation likely contributes to their development of the disease as well. Some of these people have normal body weight but high levels of visceral fat (fat that grows around the internal organs). These people may suffer from the same fat-induced inflammation as overweight people.
Smoking also causes inflammation in the body and is a significant risk factor for diabetes regardless of body weight. Chemicals in cigarette smoke injure cells, causing inflammation and preventing normal cell function.
People with chronic inflammatory diseases also have a higher risk of developing type 2 diabetes. About one-third of chronic hepatitis C patients develop type 2 diabetes, and people with rheumatoid arthritis have a 50% increased risk of developing type 2 diabetes.
Reducing your risk of developing cancer, atherosclerosis, and type 2 diabetes
Our natural immune response allows us to recover from injuries and infections, and we wouldn’t survive without it. But as you’ve learned, we only want inflammation to occur when we really need it—because chronic inflammation increases our risk of developing deadly health conditions.
The good news is that many causes of chronic inflammation are lifestyle-related and entirely controllable. So while scientists develop new drugs and treatments aimed at reducing inflammation, the best way to prevent inflammation-related conditions is undeniably to live a healthy lifestyle and avoid chronic inflammation in the first place. Check out 12 Causes of Chronic Inflammation!